5 Conceptual Site Models and Investigative Strategies
This section provides guidance to create a conceptual site model (CSM) that specifically accounts for the unique properties of TPH, allowing for a more complete understanding of the fate and transport and exposure pathways required to assess TPH risk.
Recognizing that TPH is made up of many hundreds to thousands of individual compounds that can change composition both spatially and temporally over time and may result in some risk requires an investigative strategy that considers TPH properties. Because TPH can be present at a site in a variety of forms (NAPL, dissolved, volatilized, etc.) and in a variety of media (soil, water, air), it is essential for a practitioner to understand how the TPH analytical methods vary, and when to apply them. The CSM topic will discuss various TPH analytical methods and what the results mean for both characterizing the toxicity of TPH and evaluating the degree of potential exposure to human and ecological receptors.
A CSM shows the relationship in three dimensions between contaminant sources and receptors through potential or actual migration and exposure pathways. A CSM at a site contaminated with petroleum must account for the unique properties of TPH (see TPH Fundamentals) and include an understanding of the fate and transport mechanisms that will dictate the potential exposure of receptors to TPH. The CSM should be maintained and updated as new information is collected throughout the life cycle of a project. Various styles of CSMs are useful, from text explanations to a series of figures depicting current and predicted future site conditions. A form of visualization (e.g., figures, graphs, charts, tables) that relates site conditions to receptors in a manner that lends itself to explanation of TPH data is suggested (Figure 5-1 provides an example).
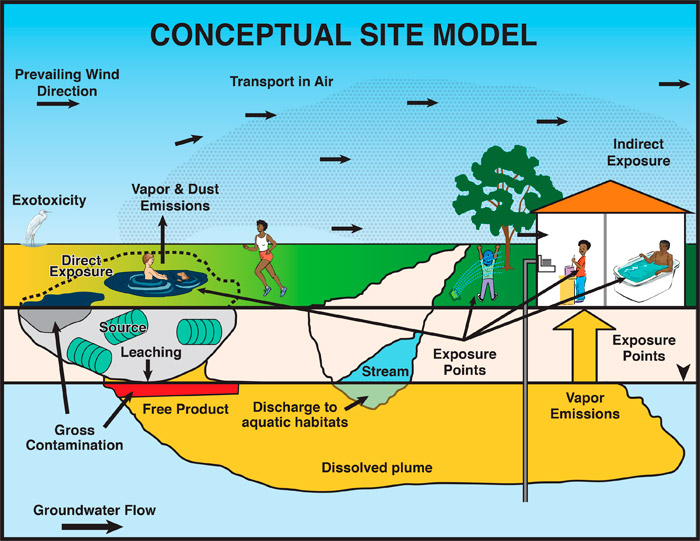
Figure 5‑1. Conceptual site model (visual depiction) showing the migration pathways of petroleum from source to receptors.
Some of the fundamental aspects of a CSM that also apply to evaluating the risk of TPH are listed below, following the typical source-pathway-receptor conceptual model shown in Table 5-1. This template can be applied in developing a site-specific conceptual site model by indicating site-specific conditions ( yes; no) as observed.
yes; no) as observed.
Other considerations (site and surrounding history, current and future land use, geology, hydrology, climate, etc.) are also important in the development of a CSM; these are covered in the prior-listed CSM references.
- Source identification (Qualitative CSM)—Identifying the type of petroleum released to the environment, adjusting TPH methods to reflect the petroleum type, identifying mixes/comingled petroleum releases using TPH analyses.
- Source area characterization, extent, distribution (Source definition)—Identifying the source of the release, areas of storage and transport, and alternate transformation phase (dissolved, gaseous states) of TPH. Identifying vadose zone, smear zone, and submerged contamination, total contaminant mass, and determining the extent of the contamination.
- Fate, transport, and attenuation mechanisms (Pathway definition)—Determining the relative influences of transport and transformation processes on potential TPH exposure pathways, considering the potential changing composition of TPH over time and space.
- Points of exposure (Receptor definition)—Identifying impacted and potentially impacted points of exposure and determining whether the composition of the TPH contamination poses a risk to receptors.
- TPH target levels—Defining the extents of TPH delineation and setting risk-based remedial goals. Remediating to published screening levels may be no more protective than remediating to calculated target levels for TPH at the source and near receptors. Using TPH data to demonstrate remediation.
- Identifying data gaps—Using indicator compounds only for an assessment or risk evaluation may leave data gaps in the CSM that can be filled with TPH data.
Table 5‑1. Conceptual site model (checklist/tabular depiction) relating petroleum sources to potential receptors through relevant pathways.
| RELEASE LOCATION—Petroleum Oil Spill/Release from Containment ☐ Product storage ☐ Pipeline/Flow Line ☐ Operations ☐ Waste Management Units ☐ Drainage Channels and Impoundments ☐ Other/Unknown |
||
| ☐ MOBILE NAPL SOURCE—Flowable Petroleum Oil (ITRC LNAPL Update Guidance) | ||
| ☐ LAND SURFACE ☐ Flow and pooling of oil at ground surface |
||
| TRANSPORT BY OVERLAND FLOW (GRAVITY-DRIVEN) ☐ Lateral terrain-directed migration of oil ☐ Lateral migration of oil carried by surface water flow (rainwater or seasonal water flow) ☐ Lateral migration of oil during tidal water cycling |
||
| TRANSFORMATION BY VOLATILIZATION ☐ Vapor evolution from oil at ground surface |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact exposure to oil at ground surface ☐ Inhalation of vapors from oil |
||
| POTENTIAL ECOLOGICAL RECEPTORS ☐ Terrestrial vegetation (herbs/grasses) ☐ Reptiles and amphibians ☐ Terrestrial invertebrates (earthworms and insects) ☐ Wildlife (birds and mammals) |
||
| SURFACE WATER/SEDIMENT ☐ Nonflowing surface water (ponds, lakes, etc.) ☐ Continuously flowing surface water (streams, rivers, etc.) ☐ Tidally influenced surface water ☐ Seasonally present (ephemeral) surface water |
☐ Freshwater ☐ Brackish ☐ Saltwater |
|
| TRANSPORT BY ADVECTION/DISPERSION (WATER-DRIVEN FLOW) ☐ Movement of oil or sheens on surface water |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact exposure to oil in water/sediments ☐ Contact exposure to oil on plants |
||
| POTENTIAL ECOLOGICAL RECEPTORS ☐ Aquatic vegetation (marsh grasses; mangroves) ☐ Reptiles and amphibians ☐ Aquatic invertebrates and fish ☐ Wildlife (birds and mammals) |
||
| UNSATURATED SUBSURFACE SOIL ☐ Vertical migration of oil between land surface and the water table |
||
| TRANSPORT BY GRAVITY-DRIVEN FLOW ☐ See page of oil into unsaturated soils |
||
| TRANSPORT BY WATER-DRIVEN FLOW ☐ Smearing of oil through downward rainwater infiltration ☐ Smearing of oil at the water table interface into unsaturated soil due to transient vertical fluctuations |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact exposure to oil in soils |
||
| ECOLOGICAL RECEPTORS ☐ Not applicable to vegetation unless oil at the surface or in the root zone; see Land Surface ☐ Burrowing animals |
||
| GROUNDWATER SATURATED ZONE ☐ Lateral migration of oil at the water table interface |
||
| TRANSPORT BY GROUNDWATER-DRIVEN FLOW ☐ Smearing and submerging of oil at the water table interface due to transient vertical fluctuations ☐ Lateral migration of oil at the water table due to transient vertical fluctuations and lateral gradients |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact/ingestion exposure to oil in abstracted groundwater (wells screened at the water table) |
||
| ECOLOGICAL RECEPTORS ☐ Not applicable unless oil is in root zone; see Land Surface |
||
| ☐ RESIDUAL NAPL SOURCE—Immobile NAPL Trapped in or on Solid Media | ||
| SURFACE SOIL ☐ Residual oil (likely weathered, lower concentrations) on soil |
||
| TRANSPORT BY DIRECT EXPOSURE ☐ No transport driver |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact, incidental ingestion, and inhalation exposure to residual oil in shallow soils ☐ Agricultural crops—ingestion of root vegetables |
||
| ECOLOGICAL RECEPTORS ☐ Impacted vegetation (dead, stressed) ☐ Degraded soil quality (oil-crusted soils) |
||
| UNSATURATED SUBSURFACE SOIL ☐ Residual oil (may be weathered, lower concentrations) in soil |
||
| TRANSFORMATION BY VOLATILIZATION ☐ Vapor evolution from impacted subsurface soils |
||
| TRANSFORMATION BY DISSOLUTION (WATER-DRIVEN FLOW) ☐ Downward migration of water-soluble oil components due to rainwater infiltration |
||
| HUMAN RECEPTOR PATHWAYS ☐ Inhalation exposure to vapors |
||
| ECOLOGICAL RECEPTORS ☐ Burrowing animals |
||
| SUBSURFACE SOIL AT WATER TABLE ☐ Residual oil (may be weathered, lower concentrations) at the water table interface |
||
| TRANSFORMATION BY DISSOLUTION (WATER-DRIVEN FLOW) ☐ Dissolution of water-soluble oil components into water ☐ Lateral transport of water-soluble oil components as a groundwater plume |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact and ingestion exposure to soluble oil constituents in abstracted groundwater (shallow water wells only; freshwater only) ☐ Contact exposure with subsurface soils during excavation |
||
| ECOLOGICAL RECEPTORS ☐ Not applicable unless oil is in root zone; see Surface Soil |
||
| SEDIMENT AND HYPORHEIC ZONE ☐ Residual oil (may be weathered, lower concentrations) in sediment |
||
| TRANSFORMATION BY DISSOLUTION ☐ Dissolution of water-soluble oil components into water |
||
| HUMAN RECEPTOR PATHWAYS ☐ Contact exposure to sediments ☐ Fish consumption |
||
| ECOLOGICAL RECEPTORS ☐ Impacted vegetation (dead, stressed) ☐ Impacted benthic organisms ☐ Reptiles and amphibians ☐ Fish and aquatic animals |
||
5.1 Source Identification (Qualitative CSM)
Petroleum contamination can originate from a range of refined products, crude oils, and condensates. The initial indications of the type of petroleum released will include where the release occurred and from what kind of infrastructure or facility (see Table 5-1).
5.2 Source Area Characterization, Extent, Distribution (Source Definition)
At the time of a release and shortly thereafter, TPH contamination will be in the form of a mobile, free-phase immiscible liquid (flowable petroleum oil) often referred to as mobile NAPL (nonaqueous phase liquid) CL:AIRE 2014; ITRC 2018. From there, the free liquid can migrate across land surfaces, to surface water, into the unsaturated soil zones, and/or down to the saturated soil zones (groundwater) through overland flow, advection, dispersion, water-driven flow, and/or groundwater-driven flow. These pathways are included in Table 5-1.
Methods for characterizing and assessing the three-dimensional extent and distribution of NAPL in the subsurface are summarized in ITRC LNAPL-3 ITRC 2018. Delineating a NAPL source can include qualitative observations such as measuring NAPL presence in monitoring wells. In addition, collecting TPH data from different media is often the best method of determining the presence of petroleum contamination (ITRC LNAPL-2) ITRC 2009a. If the type of fuel released is unknown, “bulk” TPH analyses (e.g., EPA method 8015) in the area(s) most likely to be the source of the release (based on a qualitative CSM) is often sufficient as a starting point. However, it will not be helpful in determining the type of petroleum contamination present, the degree of weathering, the presence of bioattenuation, or much information on the toxicity of the contaminated media. Furthermore, TPH data can quantify and qualitatively illustrate a petroleum release better than relying on data about individual compounds or the typical indicator BTEX compounds. Indicator compounds, such as benzene, can be absent or otherwise below levels of potential concern in contaminated media, and this can result in underestimation of risk and contaminant mass.
5.3 Fate, Transport, and Attenuation Mechanisms (Pathway Definition)
NAPL movement as a result of a release (see Table 5-1) depends on the hydrocarbon liquid bulk properties (viscosity, density, surface tension); soil properties (porosity, pore size distributions, connected pore spaces, moisture levels); and interface properties (surface tension, sorption, molecular forces). The fate, transport, and attenuation mechanisms for NAPL are discussed in more detail elsewhere Garg et al. 2017; ITRC 2009a, 2009b, 2018. A release of NAPL will transport through various mechanisms, including overland flow (gravity-driven), advection, dispersion, water-driven flow, and groundwater-driven flow.
Source zones of residual NAPL in soils and as mobile NAPL will weather and deplete over time due to dissolution, volatilization, and biodegradation (known as natural source zone depletion. These processes will result in the partitioning of TPH into various phases based on physical chemistry and deplete TPH constituents at varying rates. Therefore, the TPH composition of the NAPL will differ from the TPH composition in the dissolved and vapor phases. The maximum aqueous or vapor concentrations of the chemical constituents making up the TPH are much less than those of the pure chemicals and will be dictated by saturation limits and partitioning coefficients.
To have a robust CSM, you must further estimate how the TPH in all phases (NAPL, aqueous, and vapor) will vary both spatially and temporally. Over time, TPH constituents in soil moisture can migrate by capillary action, gravity, and infiltrating rainwater and potentially leach into groundwater. For sites where NAPL has reached the water table, TPH constituents may dissolve directly into the groundwater and migrate with the groundwater flow, creating a TPH plume. However, based on the physical chemistry of the TPH and the saturated soil environment, a TPH plume may have retarded migration compared to the groundwater itself. Similarly, NAPL that has affected surface water may dissolve and transport TPH constituents farther downgradient, depending on the flow and water chemistry characteristics of the surface water body. For vapors, TPH constituents may migrate through air-connected soil pores to the ground surface and into breathing zones both in indoor and outdoor air ITRC 2014; USEPA 2015a. Most chemical constituents degrade and attenuate with increasing distance from the original release location. In addition to measuring petroleum hydrocarbons in the environment, the presence of degradation byproducts (also called metabolites, polar intermediates) can be evidence of ongoing transformation processes.
5.4 Points of Exposure (Receptor Definition)
Human and ecological receptors may be exposed to TPH through different environmental media (soil, water, air) and through different routes of uptake (direct contact/absorption, ingestion, inhalation). These points of exposure (human receptor pathways and ecological receptor types) are also summarized in Table 5-1 for the different media. Human exposure pathways are usually associated with the mode of uptake, while ecological receptors include vegetation, invertebrates, benthic organisms, amphibians/reptiles, fish, birds, and other animals.
5.5 TPH Target Levels
Human and ecological target levels are usually set based on the local regulatory jurisdiction (see Regulatory Framework). Different TPH ranges (e.g., gasoline, diesel, oil) or fractions based on carbon number may have established target levels for some or all different types of media and relevant exposure pathways (see Risk Calculators). The relevance of media and exposure routes varies (spatially and temporally) for different compositions of TPH. Thus, site-specific target concentrations for TPH constituents might also need to be established and used in developing the CSM. The specific target levels established for TPH should be pathway-specific.
TPH target levels would be used to help delineate the extents of TPH contamination in the different media.
The uptake concentrations of TPH in vegetables and fish are extrapolations of the measured uptake of identifiable and confirmable chemicals. The only method to confirm exposure in relation to TPH target levels is through sampling and analyses. (See Sections 6.8.3 and 6.11 for more information.)
When TPH targets are established for a site and incorporated into the CSM to delineate the zones of contamination, an immediate risk evaluation can be conducted by simply showing that concentrations at the point of exposure are below target levels. Alternatively, exclusion distances or criteria can be applied to potential receptors as being at risk. For evaluating potential ecological exposures, some approaches rely on concentration-based screening criteria for specific media. For actual spills and releases, there is the additional option of evaluating a site based on observed ecological impacts relative to a nearby control site.
5.6 Compiling a CSM
The investigative strategy should focus on filling data gaps in a CSM, whether that starts from a qualitative CSM or refining or expanding upon a quantitative CSM. The appropriate sampling methods, handling procedures, data quality objectives, laboratory and field analyses, and interpretation and reporting of results should be used. TPH has specific physical, chemical, and biological behaviors that need to be considered while developing a quantitative CSM, and therefore, those unique aspects of TPH will also need to be considered in the investigative strategy.
In general, myriad decisions need to be made when compiling quantitative information on a CSM for a petroleum release site, including:
- Decisions and planning on the quantity of data and the spatial distribution of TPH data collection
- Determinations of what samples to collect from various media (including background samples) using appropriate field sampling tools and handling procedures
- Identifying which TPH analytical methods to use and request from a laboratory
5.7 TPH Data Collection Plan
As with any site assessment, multiple lines of evidence should be considered in the TPH data collection plan, including any historic site uses, monitoring data, and remediation activities. The expected nature and extent of the TPH should be based on the CSM, including the various media suspected to be impacted, compositional phases, and any historic and ongoing physical, chemical, and biological properties affecting the TPH behaviors. The CSM should then be used to inform the investigative strategy, oftentimes in an iterative manner (e.g., an evolving CSM)). Using what is known about the fate and transport of petroleum compounds, the specific TPH data to be included in the investigation strategy were summarized in the typical compositions shown in Table 5-2.
In addition to the type of TPH data to be collected, a TPH data collection plan should include several other considerations that focus on the numbers and locations of samples needed for the risk assessment, as well as supporting data.
- The amount of TPH data (defined by the number of sampling locations/depths and/or spatial density) to be collected should be sufficient, depending on how the data will be used. For more information see ITRC Geospatial Analysis guidance 2016a.
- For developing a qualitative CSM where determining simple presence/absence of TPH or delineating contamination is the goal, fewer locations/low-density sampling or screening may be sufficient as an initial indication of the release boundaries.
- For developing a quantitative CSM where characterizing fate and transport mechanisms affecting the TPH is the goal, more locations/higher density sampling may be required.
- For conducting the hazard/risk assessment establishing exposure pathway connectivity, chemical toxicity thresholds, receptor characteristics, and any other data the risk assessor may need, specific locations relevant to exposure pathways may need higher density sampling and/or specific TPH parameters (fractions, indicator compounds, specific analytes) included.
- Where and when preliminary screening data would be collected versus comprehensive data (e.g., as the CSM or risk assessment is refined)
- The media (soil, sediment, soil gas, groundwater, surface water) in which samples should be collected and the sampling locations and density for that media
- Supporting samples that should be collected in addition to the TPH data (e.g., background samples—all media, media-specific samples to determine fraction of organic carbon, pore saturation, geochemistry, natural attenuation parameters, fixed gases, toxicity testing, etc.). See Risk Calculators for information regarding what site-specific parameters are needed for each model
- Presence, toxicity, and potential risks of daughter products of TPH degradation (polar metabolites)
- Adequate sample volumes to collect, which should account for:
- Method-specific sample volume requirements (e.g., bulk vs. fractionated TPH analyses; range of fractions; and separate aliphatics from aromatics)
- Number and types of sample extractions needed (e.g., hexane, methanol, methylene chloride)
- Analysis with or without the use of silica gel cleanup (SGC), or if both SGC and non-SGC data are needed, to account for TPH degradation products
- Indicator or specific compounds (e.g., BTEX, PAHs, etc.) analyzed using different methods from TPH, or subtracted from the TPH results
- Use of a biased or nonbiased sampling scheme (see Geospatial Analysis for Optimization at Environmental Sites (ITRC GRO)).
Ultimately, the TPH data collection plan should also be informed by the sample collection and handling methods, data quality objectives (DQOs), expected TPH analyses to be conducted on the samples, and how all of the data will be used and interpreted in the risk assessment (see Data Usability, Interpretation, and Implications). Each of these sections should be reviewed as part of developing the TPH data collection plan.
5.8 Field Sampling Methods and Handling Procedures
The sample collection method will be based on the specific media. Various field screening methods are available to provide an initial characterization of a petroleum release. These initial characterization approaches (see Field Screening Methods Factsheet) can be used to help refine a qualitative CSM to become more quantitative. Furthermore, these tools can be useful to help refine the investigative strategy, including the sampling methods, analytical selection, and interpretation of data.
For soil and sediment sampling, single grab, composite, or incremental sampling methodology (ISM) ITRC 2012 can be used, based on the project objectives. For groundwater, passive sampling or no/low-purge sampling should be considered to minimize sample turbidity ITRC 2006. Likewise, the use of bailers or specific pumps, as well as the appropriate use of sample filtering in the field, should be defined in the work plan. For soil gas vapors, passivated stainless-steel (e.g., Summa) canisters or sorbent tubes are recommended, depending on the TPH carbon ranges targeted ITRC 2014. In all cases, regardless of the media sampled, field observations collected during the sampling collection event should be noted in as much detail as possible, because many of the observed conditions help to refine subsequent analytical procedures and the interpretation of the analytical results in the TPH risk assessment. Different considerations are given for:
- Sampling and handling soil and sediment
- Sampling and handling groundwater or surface water
- Sampling and handling soil gas vapors
5.8.1 Sampling and Handling Considerations for Soil and Sediment
5.8.2 Sampling and Handling Considerations for Groundwater and Surface Water
5.8.3 Sampling and Handling Considerations for Soil Gas Vapors
5.9 TPH Analytical Methods
TPH is a method-defined parameter. Many different methods have been used, or are still being used today, to quantify TPH. There are a number of widely used technical references that contain summaries of the history and types of TPH analyses TPHCWG 1997a; ATSDR 1999; CASWB 2015; ITRC 2014; API 2001. A white paper was prepared for API regarding TPH analytical methods Zemo 2016 that includes the history of analyses, analytical methods currently in use, various technical aspects of the analysis and data interpretation, and other related topics. With different methods potentially in use, the results can be tremendously variable, providing different measurements of TPH, different definitions of what is included in TPH as a measurement, and how comparable TPH data are between sites or over time. The generalized quantitation method, type of data provided, advantages and limitations, and potential applications of generalized categories of TPH analyses are summarized in Table 5-4.
Table 5‑4. General information provided by the different categories of TPH analyses
| TPH Method (Quantification) | Boiling Point/Carbon Range | Molecule Type | Subject to Including Nonhydrocarbons | QA/QC for SGC Option | Chromatogram | Potential Application |
|---|---|---|---|---|---|---|
| Bulk Gravimetric
EPA Method 1664A; Hexane Extractable Material (HEM) and Silica Gel-Treated HEM; SW846 9071* |
No | No | Yes | No | No | Not recommended for use except if required by regulatory agency (e.g., NPDES). Too little information is provided and no mechanism is available to evaluate results. |
| Bulk Infrared (IR)
ASTM D7066-04 2017 |
No | No | Yes | No | No | Not recommended. Too little information is provided and no mechanism is available to evaluate results. |
| Bulk GC-Flame ionization detector (FID)
EPA Method 8015*-based; TPH- (purgeable and extractable) TX 1005 (single pentane extraction) |
Rough | No | Yes | Yes, if using Method 3630C for SGC | Yes | Use for “total organics” analysis of bulk TPH-GRO only, because no cleanup is available.
Use for “total organics” analysis for bulk TPH unless SGC is used. Use for determining the extent of impacts. |
| Bulk GC-MS
EPA Method 8260* (quantitated using response within selected boiling point range compared to product or individual hydrocarbon standard) Missouri DNR MRBCA Guidance GC-MS TPH-DRO/ORO/RRO method according to EPA Method 8270* |
Rough | No | Yes, unless manual subtraction is done | No | Yes | Use for “total organics” analysis for bulk TPH-GRO unless manual subtraction is done.
Use for “total organics” analysis for bulk TPH-DRO/ORO/RRO, unless SGC is used. Use for determining the extent of impacts. |
| Fractionated TPH by GC-PID (photoionization detector) and/or GC-FID
Washington State Dept. of Ecology (WADOE) or MassDEP VPH/EPH (purgeable and extractable) TX 1006 (single pentane extraction) |
Detailed | Aliphatics, aromatics | Less likely | Yes | Yes | Use to obtain detailed fraction data for fate and transport calculations and/or human health risk assessments.
Representative samples can be analyzed to characterize nature of petroleum mixture. Results will not include an estimation of polar compounds. |
| Bulk GC-FID for air-phase samples
TO-3 USEPA 1984 |
Rough | No | Yes | NA | Yes | Use for “total organics” analysis for bulk TPH-GRO. FID does not provide total ion chromatograms.
Use for determining the extent of impacts. |
| Bulk GC-MS for air-phase samples
TO-15, TO-17 |
Rough | No | Yes, unless manual subtraction is done | NA | Yes | Use for “total organics” analysis for bulk TPH-GRO unless manual subtractions are conducted.
Use for determining the extent of impacts. |
| Fractionated TPH by GC/MS for air-phase samples
MassDEP APH |
Detailed | Aliphatics, aromatics | Yes, unless manual subtraction is done | NA | Yes | Use to conduct a human health risk assessment. |
| Fractionated VPH by GC/MS
MassDEP VPH |
Detailed | Aliphatics, aromatics | Yes, unless manual subtraction is done | NA | Yes | Use to conduct a human health risk assessment. |
| *most current version | ||||||
Note: Methods listed are intended to be examples of some commonly used methods. This is not an all-inclusive list of methods.
In addition to TPH, individual compound analyses are typically required to assess risk at petroleum release sites. These could include BTEX, naphthalene, MTBE, tetraethyl lead, etc., and depend on the suspected product released (e.g., crude oil vs. gasoline vs. diesel, etc.). Appendix C—States Survey shows the general differences among the states for the requirements for TPH analyses, as well as requirements for individual compounds; this is based on the State Survey conducted by ITRC in 2017. Based on best practices, it is recommended that a combination of individual compounds and TPH analysis is necessary for conducting a risk assessment at petroleum release sites, but the list of compounds can be tailored to the type of petroleum released. Some common individual compound analyses and associated analytical methods are summarized in Table 5-5.
Table 5‑5. Recommended individual compounds and analytical method
| Individual Compound(s) | Method by Matrix | Petroleum Type/Other Comments |
|---|---|---|
| Methane, oxygen, and carbon dioxide | Groundwater (GW): RSK-175
Air: ASTM D1945 or EPA Method 3C |
All petroleum types
Used to measure redox conditions and degradation indicators. |
| n-hexane | Product, Soil, Water: EPA Method 8260*
Air: TO-15/TO-17 |
Gasoline only. n-Hexane data are needed to avoid default toxicity for EC6–EC8 aliphatics fraction, as in EPA Provisional Peer-Reviewed Toxicity Values (PPRTV). |
| BTEX | Product, Soil, Water: EPA Method 8260*
Air: TO-15/TO-17 |
All petroleum types |
| MTBE, TBA | Product, Soil, Water: EPA Method 8260*
Air: TO-15/TO-17 |
Gasoline only. Can be found at trace concentrations in other products due to cross-contamination in distribution systems. |
| Naphthalene | Product, Soil, Water: EPA Methods 8260* or 8270* SIM
Air: TO-15/TO-17 |
All petroleum types
Note that EPA Method 8310 is not recommended due to potential co-elution problems. |
| 2-Methylnaphthalene | Product, Soil, Water: EPA Method 8270* SIM
Air: TO-17/TO-13A |
All petroleum types. These data are needed to avoid default toxicity value for EC12 to EC16 aromatics fraction, as in EPA PPRTV
Note that EPA Method 8310 is not recommended due to potential co-elution problems. |
| PAHs | Product, Soil, Water: EPA Method 8270D SIM
Air: TO-17/TO-13A |
Only for products heavier than diesel 2 (e.g., crude oil, marine diesel, and bunker fuel)
Note that EPA Method 8310 is not recommended due to potential co-elution problems. |
| Lead scavengers:
Ethylene dichloride (EDC), ethylene dibromide (EDB) |
Product, Soil, Water: EPA Method 8260*
Air: TO-15/TO-17 |
Pre-1997 automotive gasoline. Could potentially use Methods 8011 or 504.1/524.1 if needed for lower reporting limit (depends on state). |
| Organic (alkyl) lead (total and then speciated) | Product: GC-ECD
Soil: EPA Method 8270* SIM Water: EPA Method 8260* SIM |
Pre-1997 automotive gasoline, or any aviation gasoline.
Note that CA HML-939M is not recommended due to potential for complexation of inorganic lead onto soil organic matter. |
| *most current version | ||
5.10 Silica Gel Cleanup Method
At present, there is not a mechanism to separate hydrocarbons and metabolites for the bulk purgeable organics or gasoline range TPH.
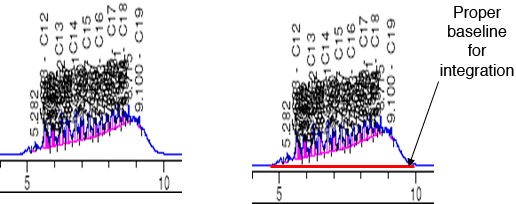
A significant and well-known characteristic of the bulk GC-based TPH analytical methods is that nonhydrocarbon compounds (such as polar metabolites from TPH degradation) can be included in the TPH quantitation because FID is a nonspecific detector. For soil/sediment and water samples, a sample cleanup method can be added for the explicit purpose of trying to remove the extracted polar organics from the bulk sample so that the TPH quantitation is more representative of the actual petroleum hydrocarbons present. An example of the effects of these sample cleanup steps is shown in Figure 5-2. Furthermore, these methods can be used to separate aliphatic from aromatic fractions in the sample. There are several substrates available for this sample cleanup, including florisil (manganese silicate), alumina, and silica gel (SG), with silica gel cleanup (SGC) being the most common.
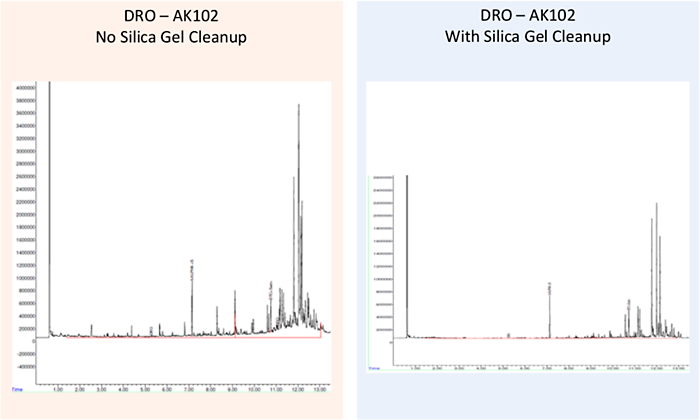
Figure 5‑2. Effects of sample cleanup on the quantitation of hydrocarbons in environmental samples.
SGC is applicable only to extractable TPH measurements, and not to gasoline range TPH.
For sites contaminated with petroleum other than, or in addition, to gasoline, the first potential option would be to analyze samples using bulk extractable carbon range TPH with and without SGC to separate the bulk hydrocarbon and bulk polar (assumed to be metabolites) portions. In this way, the extractable metabolites would not be included as TPH and could otherwise be evaluated separately. This can be done by subtracting TPH concentrations derived with SGC from the bulk TPH concentrations without SGC. The use of SGC can also supplement information evaluating the different risks between TPH and metabolites using other methods, such as chronic toxicity evaluation as conducted for NPDES permit requirements (see Whole Effluent Toxicity Testing).
The distinction and separation of polar and nonpolar hydrocarbons are particularly important for groundwater samples because hydrocarbons have relatively low solubility and are not typically present in groundwater at elevated concentrations while polar compounds have relatively higher solubility and can be present in groundwater at relatively higher concentrations. Some additional points to consider associated with the decision to employ the sample cleanup approach include:
- For purgeable constituents, or air-phase samples, there is no cleanup step available to separate the hydrocarbons and nonhydrocarbons in the sample due to the nature of analysis for volatiles. Therefore, careful evaluation of the sample chromatogram and discrete-constituent data is necessary.
- These methods are not acceptable for typical gasoline range hydrocarbons because the volatiles in this fraction would be lost during the extraction and extract handling procedures.
If SGC is used, it is also critical that one or more representative background sample(s) are collected to assist in differentiating the naturally occurring oxygenated compounds and those originating from the release.
Nonhydrocarbons can also be present in environmental samples due to (1) naturally occurring organics (e.g., humic acids, some plant waxes, plant essential oils), (2) sampling or lab artifacts (e.g., phthalates, equipment lubricants), or (3) nonpetroleum-related chemicals (e.g., solvents, creosote) Zemo, Bruya, and Graf 1995; Uhler, Stout, and McCarthy 1998. SGC may not be very effective on certain types of nonpetroleum organics if the compound has relatively lower polarity. For example, natural hydrocarbons like plant waxes, if present, would not be removed by SGC and could be mistaken for petroleum hydrocarbons. Several examples of constituents measured as petroleum hydrocarbons but found in nonpetroleum sources are shown in Figure 5-3.
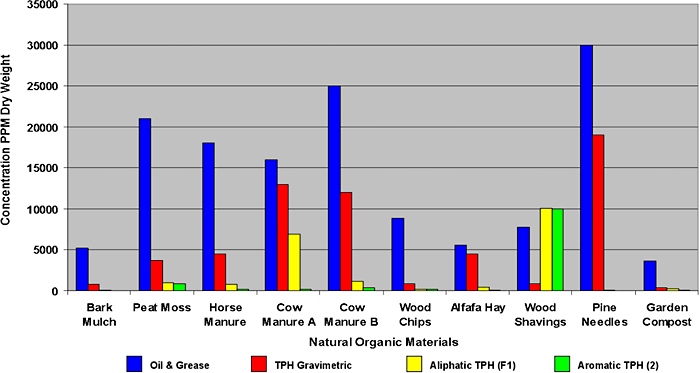
Figure 5‑3. Concentrations of petroleum hydrocarbons in natural organic materials as measured by TPH analytical methods.
(Source: G. Douglas et. al., NewFields Environmental. 2002)
Regardless of which SGC method is used, laboratory control samples should demonstrate that the polar compounds have been adequately removed using a polar surrogate such as capric acid added to all samples. Similarly, laboratory QA/QC samples should also be run using known hydrocarbon spikes such as fresh diesel or individual hydrocarbon compounds to demonstrate that hydrocarbons can be sufficiently recovered in the cleaned extract. The laboratory should also use standards that have undergone the same cleanup technique to calibrate the gas chromatograph before analysis.
5.11 Reporting, Data Qualification, and Chromatograms
TPH results should be reported as detected only if the concentration is above the reporting limit (RL). Furthermore, it is imperative that the RLs (and not the method detection limits [MDLs]) for each method are evaluated versus the project screening criteria prior to submitting samples to the laboratory. The RLs should be below the project screening criteria to ensure achievement of project objectives. Lower screening criteria could result in the need for a different method, a method modification to lower the RLs (e.g., selective ion monitoring for the indicator compounds), or maybe a different laboratory.
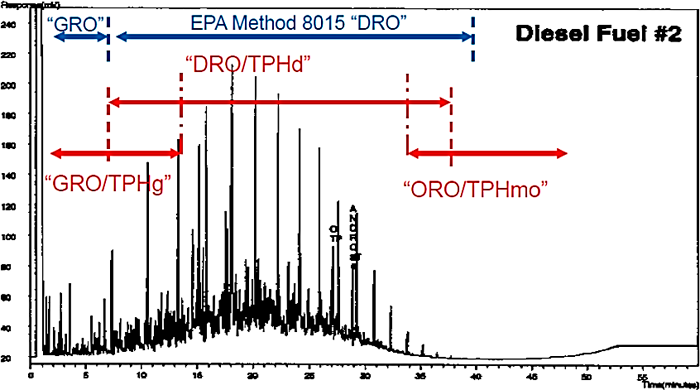
In addition to a numerical data package, TPH chromatograms can be obtained for most TPH methods (see Table 5-4). Chromatograms should be requested and reviewed to account for changes in the patterns due to various weathering processes and partitioning of hydrocarbons, depending on the medium sampled. For example, the chromatographic patterns of product samples may appear similar to patterns obtained from soil samples at the same site because the soil samples likely contained trapped or sorbed product under the same weathering conditions. Conversely, the partitioning of certain hydrocarbons to the water or vapor phases results in chromatographic patterns that are far different from the product. For additional information on chromatograms, see the Chromatograms: A Wealth of Information Fact Sheet.
5.12 Data Usability, Interpretation, and Implications
When TPH data are collected, its usability has to be assessed, oftentimes by an expert with an understanding of the effects of the various sample collection methods, handling, and analytical steps taken to generate the data. The representativeness of traditional “discrete” sampling methods versus more recent “ISM-type” sampling methods is discussed in ITRC 2012 Brewer, Peard, and Heskett 2017a, 2017b. Additional data quality evaluations that may need to be considered include (found under Read more at bottom of page):
- Potential Effects of Holding-Time Exceedances on TPH Results
- Potential Effects of Blank Detections on TPH Data Interpretation
- Potential Effects of Laboratory Control Sample Results on TPH Data Interpretation
- Potential Effects of Surrogate Recoveries on TPH Data Interpretation
- Potential Effects of Matrix Spike (MS)/MS Duplicates (MSD) on TPH Data Interpretation
- Evaluating and Interpreting Breakthrough
- Potential Effects of Co-Eluting Contaminants on TPH Results
- Avoiding Double Counting of Indicator Compounds in Fractionated TPH Data
- Potential Issues Associated with TPH Chromatograms
- Evaluating Potential Uncertainty in TPH Data
Once the data have been determined to be representative of site conditions and usable, the interpretation and implications of the data for a specific petroleum release, the associated CSM, and subsequent TPH risk assessment can begin. Some specific examples include:
- The ratio of bulk-to-SGC TPH results can give important information relevant to the CSM about the degradation stage of petroleum and zonation HIDOH 2017 in the dissolved plume. For example, a bulk-to-SGC TPH ratio of 1 is associated with fresher, more un-degraded material, but there is also potential misinformation associated with data generated using SGC (see Silica Gel Cleanup Method and the Silica Gel Cleanup Fact Sheet). A high bulk-to-SGC TPH ratio may indicate a weathered product.
- If nonhydrocarbons are also found upgradient or cross-gradient of the source area, they are typically a result of natural organics, ambient organics unrelated to the petroleum source, or lab/equipment contamination. If the nonhydrocarbons are found only in samples within and downgradient of a biodegrading petroleum source area, and not in the upgradient or cross-gradient samples, they are most likely metabolites and confirmation of ongoing transformation of TPH.
- Chromatograms are useful for forensic (“fingerprint”) analyses to determine product type, degree of weathering and degradation, relative age dating, and general environmental forensic characteristics.